November 07, 2024 – According to Bloomberg, sources familiar with the matter have revealed that Precision Neuroscience, a brain-computer interface company, is seeking to raise 100millioninfunding.Thecompanyhasalreadysecured93 million, resulting in a valuation of approximately $500 million. Precision is positioned as a rival to Elon Musk’s Neuralink.
This new infusion of funds provides Precision with additional financial backing to compete with companies like Neuralink and Science. To date, Neuralink has raised over 685million,whileSciencehassecured150 million. A Precision spokesperson declined to comment on the matter. However, a document from the U.S. Securities and Exchange Commission indicates that the company has applied to raise up to $150 million.
Precision was founded in 2021 by neurosurgeon and Neuralink co-founder Ben Rapoport, along with CEO Michael Mager. Last year, the company raised $41 million.
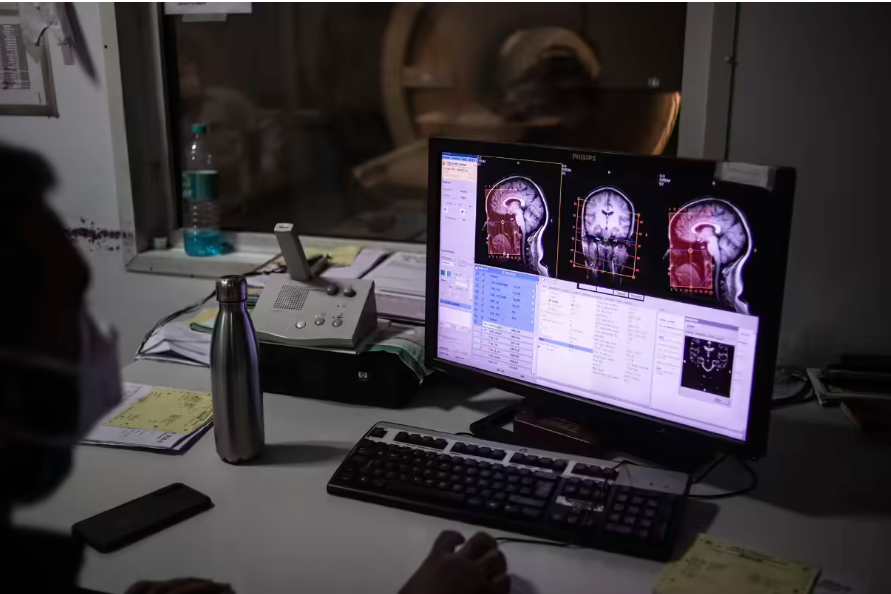
The company’s device, known as the “Layer 7 Cortical Interface,” draws inspiration from the six-layer structure of the cerebral cortex. It represents a middle ground between invasive and non-invasive brain-computer interface technologies. The device requires surgical incision of the skull but does not penetrate deep into the brain tissue, instead resting on the surface of the brain. Precision claims that this approach avoids damage to brain tissue, unlike devices from companies like Neuralink that need to be placed within the brain tissue.
Currently, Precision’s device has not received regulatory approval but is undergoing testing. The company has implanted its device in several patients, albeit temporarily during surgical procedures primarily aimed at achieving other therapeutic goals, such as removing brain tumors. With the consent of the patients, the Precision team positions its “Layer 7 Cortical Interface” to detect neural signals during these surgeries.
Precision has applied to the U.S. Food and Drug Administration (FDA) for approval of a version of its device for temporary monitoring in hospital settings. The company expressed its hope to bring this device to the market next year, providing a source of revenue for the startup. Simultaneously, Precision is developing another device: a permanent implant for treating paralysis. If successful, this device is still several years away from approval and commercial deployment.